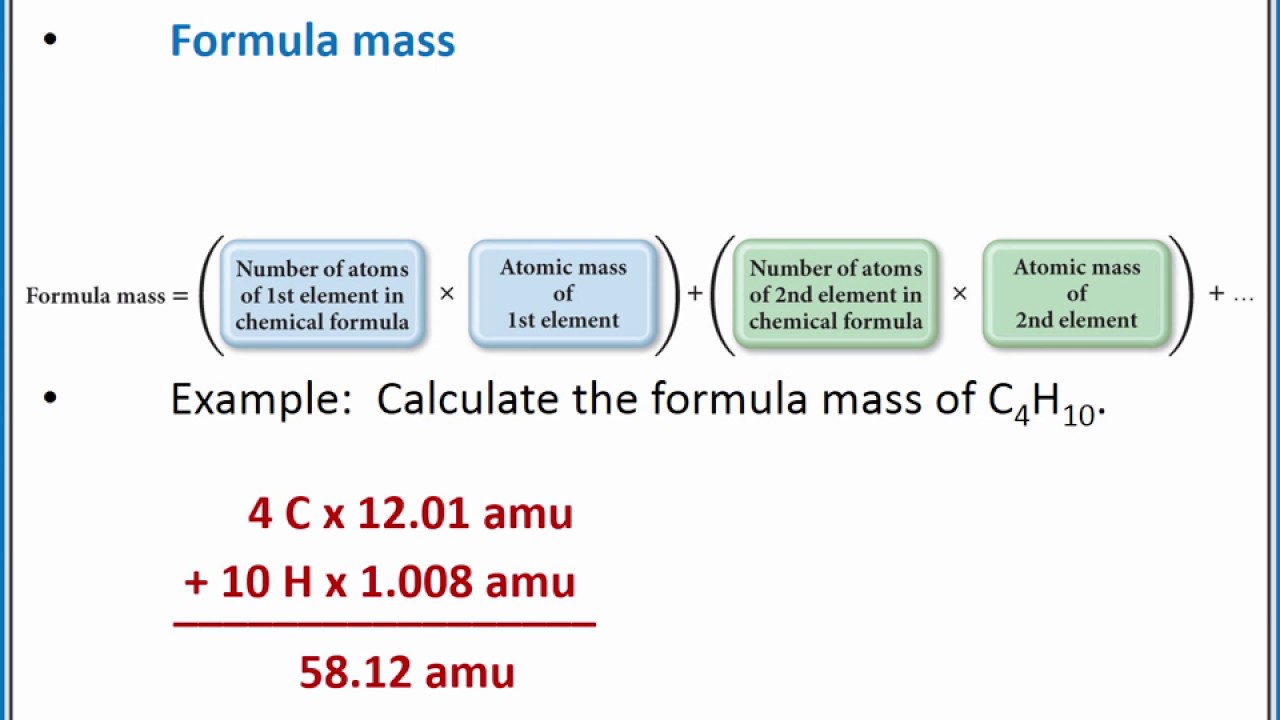
Let us start by calculating the formula mass of sodium chloride NaCl. The formula mass is obtained by adding the masses of each individual atom in the formula of the compound.
 Chemistry 101 Calculating The Formula Mass From A Chemical Formula Youtube
Chemistry 101 Calculating The Formula Mass From A Chemical Formula Youtube
The atomic masses of C H and O are 12 gmol 1 gmol and 16 gmol respectively.
What is the formula mass of a compound. 3 реда The relative formula mass of a compound is calculated by adding together the relative atomic. The empirical formula mass is 30026. Solution The formula for this compound indicates it contains Al 3 and SO 4 2 ions combined in a 23 ratio.
The formula mass is calculated by adding up all the atomic masses for every atom in the formula. Number of atoms of sulfur. Formula weight is given in atomic mass units amu.
Following the approach outlined above the formula mass for this. The empirical formula of a compound is Solution If percentage are given then we are taking total mass is 100 gramsSo the mass of each element is equal to the percentage givenMass of S 270 gMass of O 134 gMass of Cl 596 gMolar mass of S 32 gmoleMolar mass of O 16 gmoleMolar mass of Cl 355 gmoleStep 1. Use the periodic table to determine the atomic mass of each element in the molecule.
Its easy to find the molecular mass of a compound with these steps. Based The subscripts in a formula represent the number of Empirical Formula for a carbon and Hydrogen compund Molecular formula empirical formula X N N small a compound of carbon and hydrogen was found to have A compounds empirical formula is C2H5. Determine the molecular formula of the molecule.
What is the formula mass amu of this compound. Formula Mass for Covalent Substances For covalent substances the formula represents the numbers and types of atoms composing a single molecule of the substance. Let us start by calculating the formula mass of sodium chloride NaCl.
The formula mass is obtained by adding the masses of each individual atom in the formula of the compound. 4 Emperical formula SO4 As the molar mass given 19246 molecular formula - SO4n Therefore n x 32 4 x 16 19246 n 2 So molecular formula is S2O8. Because a proper formula is electrically neutral with no net electrons gained or lost the ions can be considered atoms for the purpose of calculating the formula mass.
The formula mass of a covalent compound is also called the molecular mass. To calculate the formula mass of a compound you not only need to know its formula but you must also be able to interpret the symbols numbers and brackets in the formula. This question doesnt make sense Calcium chloride is an ionic compound with a formula mass of 11098.
Oxygen 333832. Multiply each elements atomic mass by the number of atoms of that element in the molecule. The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units.
A compound has a molar mass of 12322 gmol. Your answer should have six significant figures. A compound contains 40 carbon 67 hydrogen and 533 oxygen.
What is the formula mass of a compound with 2 aluminum atoms 3 sulfur atoms and 3 oxygen atoms in atomic mass units. 100 g of the compound will contain 40 g C 67 g H and 533 g O. What Is The Formula Mass Of A Compound With 2 Carbon Atoms And 3 Oxygen Atoms In Atomic Mass.
The formula mass of a molecule also known as formula weight is the sum of the atomic weights of the atoms in the empirical formula of the compound. 4164 1. The empirical formula is CH2O.
The number of moles of C 12 g40 g. Formula Mass and the Mole Concept Calculate formula masses for covalent and ionic compounds Define the amount unit mole and the related quantity Avogadros number Explain the relation between mass moles and numbers of atoms or molecules and perform calculations deriving these. Samples of CaCl2 are crystals comprised of layers of calcium.
The formula mass of a molecule or a compound is the sum of the atomic weights of the atoms in the empirical formula. For purposes of computing a formula mass it is helpful to rewrite the formula in the simpler format Al 2 S 3 O 12. What is the bond order of C2 provided the following electron configuration.
Therefore the formula mass may be correctly referred to as a molecular mass. Because a proper formula is electrically neutral with no net electrons gained or lost the ions can be considered atoms for the purpose of calculating the formula mass. 666216 1043.
The units of formula mass atomic mass unit amu.
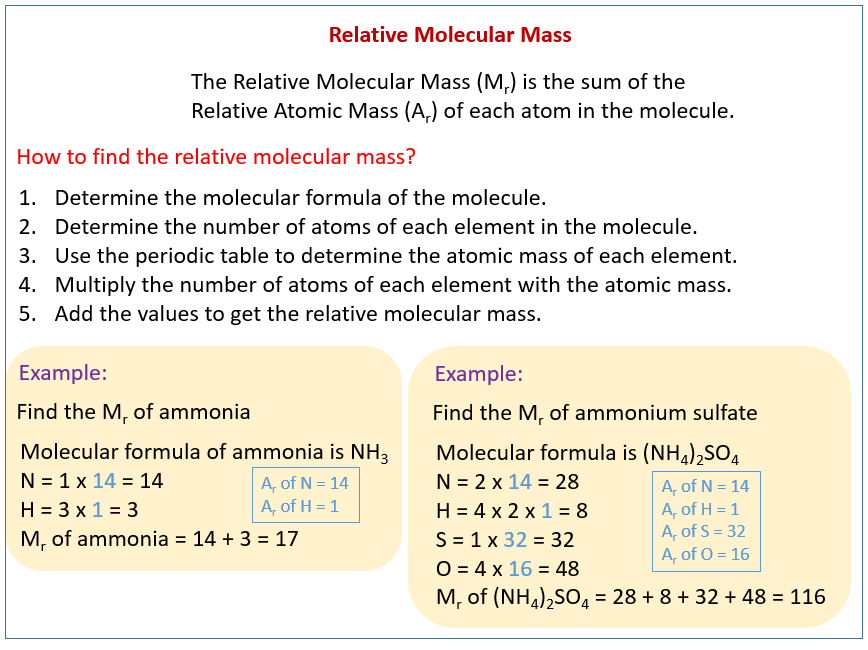 Relative Molecular Mass Relative Formula Mass Video Lessons Examples And Solutions
Relative Molecular Mass Relative Formula Mass Video Lessons Examples And Solutions
 What Is Molar Mass Definition Formula Examples Video Lesson Transcript Study Com
What Is Molar Mass Definition Formula Examples Video Lesson Transcript Study Com
 How To Calculate Molar Mass Practice Problems Youtube
How To Calculate Molar Mass Practice Problems Youtube
:max_bytes(150000):strip_icc()/GettyImages-175532236-c614b233b7e84d5487cad8b280f365a4.jpg) How To Find The Molecular Mass Of A Compound
How To Find The Molecular Mass Of A Compound
 What Is Molar Mass Definition Formula Examples Video Lesson Transcript Study Com
What Is Molar Mass Definition Formula Examples Video Lesson Transcript Study Com
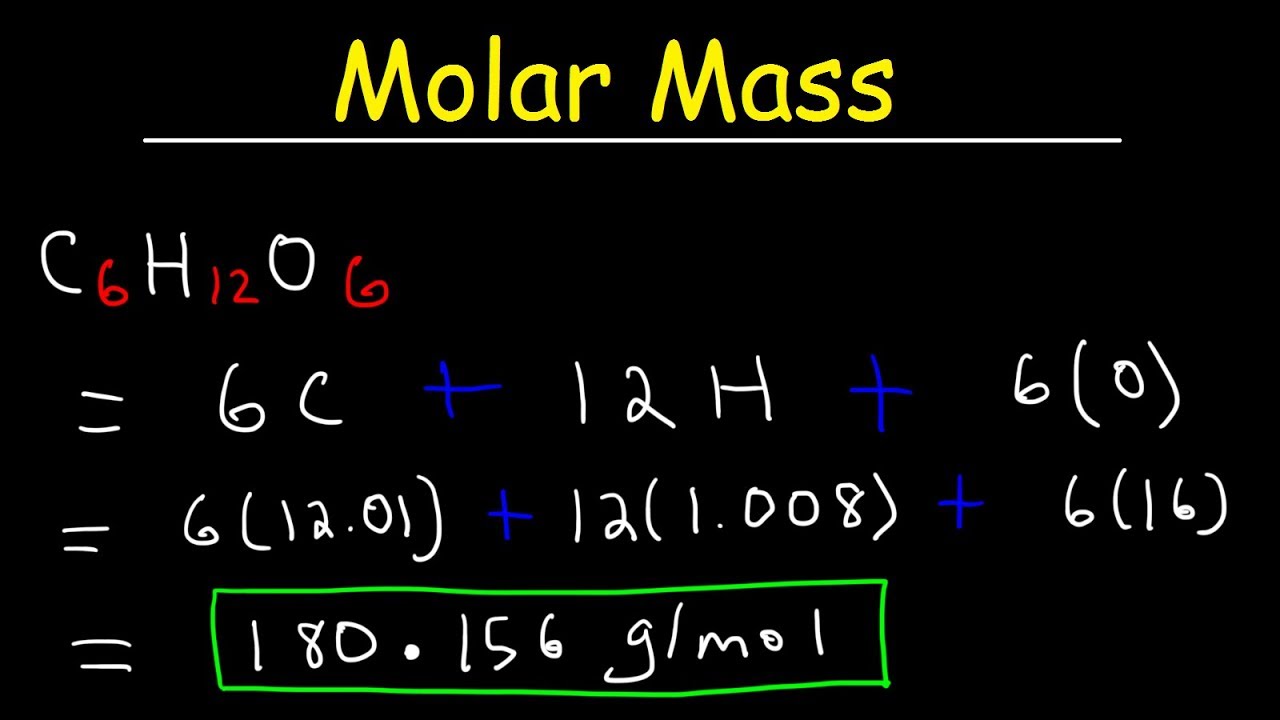 How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
 Molar Mass Of Compounds Youtube
Molar Mass Of Compounds Youtube
 Calculating The Formula Mass Of A Compound Activity
Calculating The Formula Mass Of A Compound Activity
 Chemistry Calculations Learning Intentions 1 How Can We Work Out The Formula Mass Of A Chemical 2 What Is A Mole 3 How Can We Use Formula Mass And Ppt Download
Chemistry Calculations Learning Intentions 1 How Can We Work Out The Formula Mass Of A Chemical 2 What Is A Mole 3 How Can We Use Formula Mass And Ppt Download
Popular Posts
-
Most of the agricultural societywas largely supported by the feudal system social hierarchy. The Feudal System was introduced to England fol...
-
He ruled from 5 April 1462 to 27 October 1505 he was known as Ivan the Great and was a Grand Prince of Moscow. 29 October 1603 Moscow Russia...
-
Indian National Congress 1885. N the official name for Congress3 Collins English Dictionary Complete and Unabridged 12th Edition 2014 Harper...
Featured Post
eyes but cannot see verse
83 Bible Verses about Have Eyes But Do Not See . “Son of man, you dwell in the midst of a rebellious house, who have eyes to see, but ...

ads
