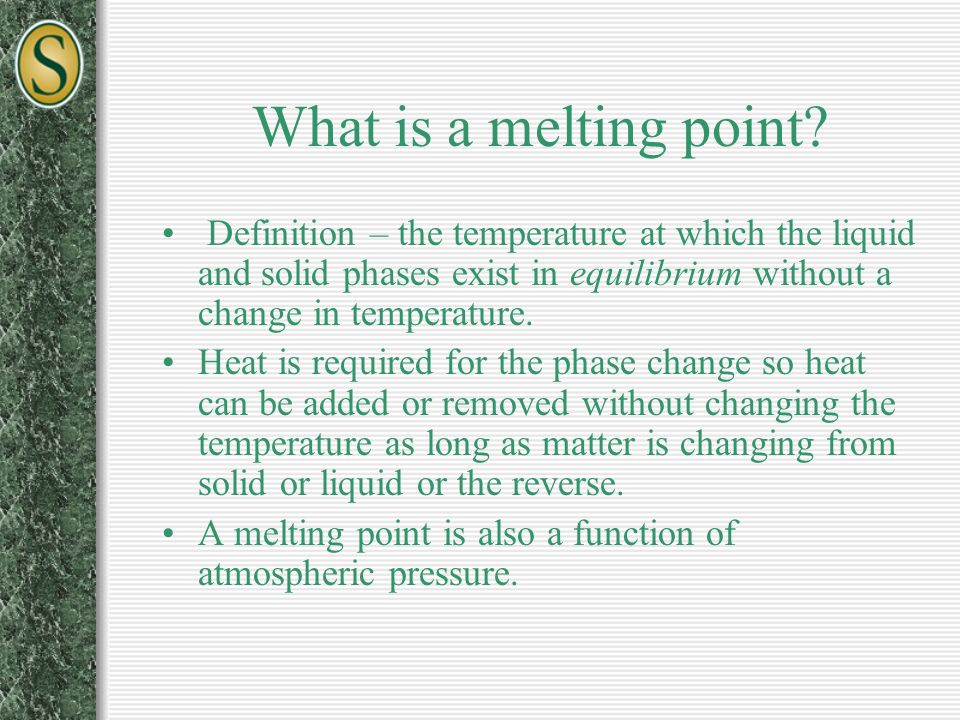
The temperature at which liquid and solid coexist in equilibrium. Melting point depends on pressure so it should be specified.
 What Is Melting Point Definition Range Determination Video Lesson Transcript Study Com
What Is Melting Point Definition Range Determination Video Lesson Transcript Study Com
Normally standard pressure is taken as 1 atmosphere.
Melting point definition chemistry. Melting change of a solid into a liquid when heat is applied. Melting point Solid state Liquid state Boiling point. The term applies to pure liquids and solutions.
Also the freezing point. It is the temperature at which the solid phase changes to the liquid phase. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid form.
Melting is the process by which a substance changes from the solid phase to the liquid phase. Melting point temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. Definition of melting point - Chemistry Dictionary Definition of melting point 1 The temperature at which liquid and solid coexist in equilibrium.
2 The temperature at which a solid changes to a liquid. Melting point is the temperature at which a solid becomes a liquid at normal atmospheric pressure. Melting occurs when the internal energy of a solid increases usually through the application of heat or pressure such that the molecules become less ordered.
Melting point is a characteristic property of solid crystalline substances. The most comprehensive Melting point Definition is The temperature at which liquid solid form of a substance co-exits in equilibrium under given pressure is called Melting point. Some materials like metals glass and other substances which are solid at room temperature have a high melting point.
Melting Point Definition The melting point of any given substance is the temperature at which the substance experiences melting or the moment that the substance changes from a solid into a liquid. As with boiling points the melting point of a solid is dependent on the strength of those attractive forces. At its melting point the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid.
Melting is also known as fusion although this term has several meanings. The melting point is the temperature at which a solid changes into a liquid. An impure solid generally melts over a range of temperatures below the melting point of the principal component.
As with boiling points the melting point of a solid. Melting Point and Freezing Point. A melting point is a useful indicator of purity as there is a general lowering and broadening of the melting range as impurities increase.
In a pure crystalline solid this process occurs at a fixed temperature called the melting point. Pressure of 760 mm of Hg Boiling point Liquid state Gaseous state. As heat is applied to a solid its temperature will increase until the melting point is reached.
At its melting point the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. The melting point is the temperature at which a solid changes into a liquid. A more specific definition of melting point or freezing point is the temperature at which the solid and liquid phases of a substance are in equilibrium at a specified pressure normally taken to be atmospheric unless stated otherwise.
Also the freezing point. Definition of melting point. The constant temperature at which a liquid becomes gas upon absorbing heat under normal pressure is called as the boiling point of that liquid.
More heat then will convert the solid into a liquid with no temperature change. Pure crystalline solids have a characteristic melting point the temperature at which the solid melts to become a liquidThe transition between the solid and the liquid is so sharp for small samples of a pure substance that melting points can be measured to 01 o C. The melting point of solid oxygen for example is -2184 o C.
Melting point determination is the thermal analysis most frequently used to characterize solid crystalline materials.
Popular Posts
-
Most of the agricultural societywas largely supported by the feudal system social hierarchy. The Feudal System was introduced to England fol...
-
He ruled from 5 April 1462 to 27 October 1505 he was known as Ivan the Great and was a Grand Prince of Moscow. 29 October 1603 Moscow Russia...
-
Indian National Congress 1885. N the official name for Congress3 Collins English Dictionary Complete and Unabridged 12th Edition 2014 Harper...
Featured Post
eyes but cannot see verse
83 Bible Verses about Have Eyes But Do Not See . “Son of man, you dwell in the midst of a rebellious house, who have eyes to see, but ...

ads
